#38-Cell and Gene Therapy Today
Stay up to date with the latest developments in your field with our weekly digest of industry news and research articles.
I’m Pedro Silva Couto, and this is Cell and Gene Therapy Today. Here I will be summarising key research papers within the field, with a special focus on the manufacturing processes used.
CGT News this week:
🧬 FDA Approves First Human Mesenchymal Stromal Cell (hMSC) Therapy for Pediatric GVHD (news here).
The US FDA has approved Mesoblast's Ryoncil (remestemcel-L-rknd) for treating steroid-refractory acute graft-versus-host disease (SR-aGVHD) in children two months and older. This marks the first mesenchymal stromal cell (MSC) therapy approved by the FDA. SR-aGVHD is a severe complication post allogeneic hematopoietic stem cell transplantation, often leading to significant morbidity. Ryoncil's approval offers a new therapeutic option for this vulnerable patient population, highlighting the potential of MSC therapies in addressing complex immune conditions. Mesoblast focuses on the next product candidate, REVASCOR® (mesenchymal precursor cells), for advanced chronic heart failure.
⚠️ Alofisel Withdrawn from EU Market Due to Lack of Efficacy
(news here).
Alofisel, a treatment for complex anal fistulas in adults with Crohn’s disease, has been withdrawn from the European Union market. Recent study data failed to demonstrate its efficacy over placebo, concluding that its benefits no longer outweigh the associated risks. Consequently, the marketing authorisation holder has decided to cease its availability in the EU. Healthcare professionals are advised to discontinue its use and consider alternative treatments for affected patients.💰 Tessera Therapeutics Secures $50M Investment for Sickle Cell Gene Therapy
(news here).
Tessera Therapeutics has received funding from the Bill & Melinda Gates Foundation to advance its in vivo gene therapy program for sickle cell disease (SCD). Utilising their proprietary Gene Writing™ technology, Tessera aims to correct the sickle mutation through a single intravenous administration, potentially offering a curative treatment without complex procedures. This investment underscores the commitment to developing accessible genetic medicines for SCD patients globally.🧬 Astellas and Sangamo Entered an Agreement for Neurological Gene Therapies, Starting with $20M (news here).
Astellas Pharma Inc. has entered into a license agreement with Sangamo Therapeutics to utilize Sangamo's proprietary neurotropic adeno-associated virus (AAV) capsid, STAC-BBB, which has demonstrated effective blood-brain barrier penetration in nonhuman primates. Sangamo will receive a $20 million upfront license fee and is eligible for up to $1.3 billion in additional fees and milestone payments across all five potential targets, along with tiered royalties on net sales. This partnership aims to advance the development of intravenous gene therapies for neurological diseases, leveraging Sangamo's delivery technology and Astellas' expertise in gene therapy research and development.🇦🇺 The Australian Government Invests $18.5 Million in Biomedical Startups in the Areas of Blindness, Cancer, and Childbirth Practices (news here).
The Australian Government is investing $18.5 million to support eight startups commercialising medical research (Mirugen Pty Ltd, OncoRes Medical, Baymatob Operations, Amplificare, Currus Biologics, Kinoxis Therapeutics, Micromune Therapeutics and SeeTreat). The funding aims to accelerate the development of new treatments and technologies, including gene therapies for blindness, cancer detection devices, and software to improve childbirth safety. This initiative reflects Australia's commitment to translating innovative research into practical healthcare solutions.
CGT Research this week:
CAR-T Manufacturing
24-Hour CAR-T Manufacturing Enhances T Cell Quality Without Compromising Cytotoxicity but Transduction Levels Fail to Impress (article here).
Over 7 CAR-T cell therapy products have been approved by the US FDA; however, the lengthy manufacturing processes can delay treatment, compromising patient access. This study presents a novel, semi-automated, 24-hour manufacturing workflow developed by Thermo Fisher Scientific employing the CTS DynaCellect System. By reducing ex vivo culture time and leveraging tools such as the G-rex or the CTS Rotea, this approach preserves naïve/stem-cell-like T cells, critical for long-term antitumor activity. The process integrates T-cell isolation, activation, and lentiviral transduction, yielding a functional product in just one day. Experiments were conducted using leukopaks from three healthy donors, and these were the main messages from the article:
The 24-hour process retained a higher percentage of naïve and stem-like T cells compared to the conventional 7-day workflow, resulting in a less differentiated product.
CAR-T cells produced via the expedited process proliferated faster after thawing, expanding 9.3-fold within four days, compared to a 4.8-fold expansion for the 7-day process. This comparison highlights the post-thaw proliferative advantage of less differentiated cells produced in the 24-hour workflow.
Cytotoxic assays showed enhanced target cell killing and higher IFN-γ secretion in the 24-hour CAR-T product, reflecting greater potency and functionality (Figure 1).
Transduction efficiency, measured as CAR expression, was comparable between the 24h and 7-day process (in the 10% ballpark), with the authors describing a “pseudo-transduction” stage overestimating CAR expression 24 hours after transduction.
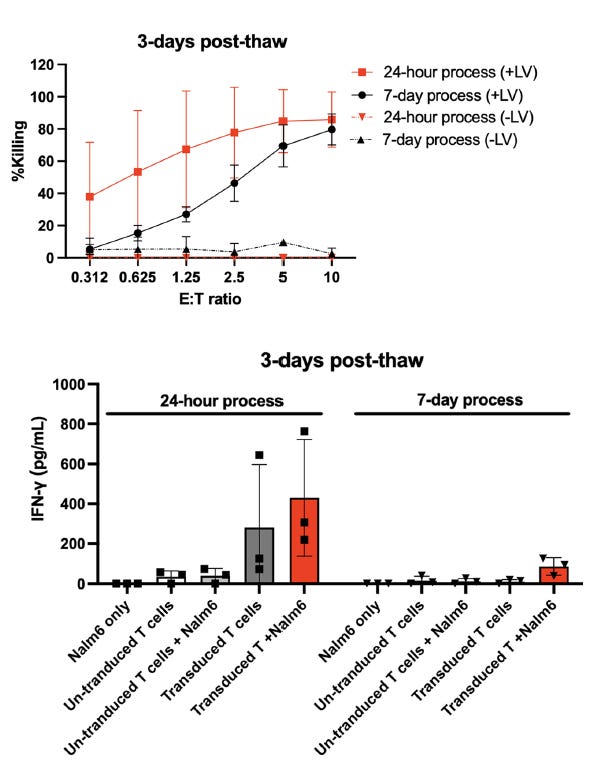
This study demonstrates the feasibility of manufacturing CAR-T cells in 24 hours while maintaining high functionality and reducing T cell exhaustion. The expedited workflow can improve patient access, lower production costs, and enhance therapeutic outcomes. Future research should evaluate clinical efficacy and address challenges related to pseudo-transduction and viral particle removal. These findings pave the way for decentralised and more accessible CAR-T manufacturing models.
hMSC-based therapy for immune disorders
Umbilical Cord-Derived MSCs: A Safe Step Forward for Severe SLE Treatment (article here).
Systemic lupus erythematosus (SLE) is a challenging autoimmune disease with limited treatment options for patients refractory to standard therapies. This study aims to fill this gap by investigating the safety of allogeneic umbilical cord tissue-derived human mesenchymal stromal cells (UCT-hMSCs) for severe SLE through a dose-escalation phase 1, open-label clinical trial (EudraCT2017-001400-29). Eight patients received intravenous infusions of 2 × 10⁶ or 4 × 10⁶ injections per kg. The study prioritised monitoring severe adverse events (SAEs) within 10 days of infusion and assessed clinical responses over a year. Here are the main messages to take home:
No treatment-related severe adverse events were reported during the 10-day post-infusion monitoring or 12-month follow-up.
Median SELENA-SLEDAI scores decreased by 7 points, suggesting clinical efficacy/improvement.
Temporary increases in regulatory B cells were observed, correlated with therapeutic response.
Clinical response was sustained in 50% of patients at 12 months.
UC-MSCs demonstrated a strong safety profile, providing a potential alternative for refractory SLE patients. The findings support further exploration of UC-MSCs in randomised placebo-controlled trials to confirm efficacy and refine dosing strategies. Broader implications include leveraging cell-based therapies to target other autoimmune diseases where current treatments fall short. The authors hypothesised that UCT-hMSCs exert their therapeutic effect by modulating pathogenic B cells and enhancing regulatory B cells, promoting immune balance and reducing autoimmunity. Furthermore, the study highlights improved biomarkers needed to predict patient responses and ensure precise patient selection for future trials. Regarding its manufacturing method, the cells were isolated from the tissue using an enzymatic and mechanical process. After the initial expansion step in monolayer, these cells were further expanded in an Xpansion-50 bioreactor in a serum-free medium to create a working cell bank. Upon patient enrollment, the cells were thawed and further expanded in five-layer cell stacks. After this stage, the product was formulated in a NaCl-based solution containing 0.5% of human albumin, ready for administration into each patient.
If you found this newsletter valuable, please consider signing up or sharing it with someone who would benefit from it!
My current scientific mission is to establish a scalable CAR-T cell manufacturing process using non-viral methods. If you would like to know more about this, please reach out! You can find more about my research on my Google Scholar or LinkedIn page.
Last but not least, this content was only possible to produce with the sponsorship of celltrials.org, the leading online portal tracking the clinical trial landscape of cell and gene therapy products. They have data packages on CAR-T, hMSCs, Extracellular Vesicles, Cell-Free products, and in vivo gene therapy products (visit celltrials.org).



